Honored Distinguished Colleagues, Precious Academicians and Our Research Professionals,
A warm welcome to the Euro Pharmacovigilance 2020...!!
Do Mark your inordinate presence at 2nd European Conference on Pharmacovigilance and Drug Safety Webinar on September 21-22, 2020.
Meet your target audience with members from around the world focused on learning about Pharmacovigilance and Drug Safety, this is your single best opportunity to reach the largest assemblage of participants from the Pharma and medicinal community,
The conference would be focusing on Pharmacovigilance and Drug safety with the theme “COVID-19 Challenges for Pharmacovigilance and Drug Safety ".
We welcome you to Webinar and hope the current year's gathering will challenge and motivate you, and result in new learning, joint efforts, associations and some more...
Regards,
Organizing Committee Members
Euro Pharmacovigilance 2020.
We warmly invites all the participants across the world to “2nd European Conference on Pharmacovigilance & Drug Safety” webinar scheduled on September 21-22, 2020
Across the theme “COVID-19 Challenges for Pharmacovigilance and Drug Safety “
This conference provides a forum for interaction among attendees with discussion involving discovery of a new drug, Challenges in drug development. Which includes a large range of prompt Keynote presentations, Poster presentations, Oral presentation, Exhibitions, Symposiums & Workshops? It's a right platform for researchers, scientists and delegates to share their expertise, poster collaborations across business and domain, and valuate rising technologies across the globe.
About conference
Euro Pharmacovigilance 2020 Meet your target audience with members from around the world focused on learning about Pharmacovigilance and Drug Safety , this is your single best opportunity to reach the largest assemblage of participants from the Pharma and medicinal community, Find out how innovators are closing the gap between product development and adoption, Better identify cyber threats and reduce information security risks, Debate the potential to greater cross-industry and cross-sector collaboration in the race for faster, cheaper, better cures. Conduct demonstrations, distribute information, meet with current and potential Scientists, make a splash with a new research, and receive name recognition at this event Euro Pharmacovigilance 2020. World-renowned speakers, the most recent techniques, tactics, and the newest updates in Pharmaceutical Sciences fields are hallmarks of this conference.
Target Audience:
-
Pharmacovigilance Students, Scientists
-
Pharmacovigilance Researchers
-
Pharmacovigilance Faculty
-
Medical Colleges
-
Pharmacovigilance Associations and Societies
-
Business Entrepreneurs
-
Training Institutes
-
Software Developing Companies
-
Manufacturing Medical Devices Companies
Pharmacovigilance and Drug Safety
Pharmacovigilance is the science and exercises identifying with the discovery, evaluation, comprehension and counteractive action of unfavourable impacts or some other pharmaceutical related issue. The main aim of pharmacovigilance is to provide complete and clear information related to drug safety and the several risks and benefits associated with them. Pharmacovigilance can help in providing information about unintended and severe adverse events which could not be provided by clinical trials involving in-vivo method.
-
Significance of pharmacovigilance
-
Pharmacovigilance and healthcare system
-
Pharmacovigilance legislation
-
Adverse Event Reporting
Clinical Trials
Clinical trials which show medical approaches work best for certain illnesses or groups of people. Clinical trials related to drugs. They are the primary way that researchers find out if a new treatment, like a new drug or diet or medicinal device is safe and effective in people. Clinical trials advance through four phases to test a treatment, find the appropriate dosage, and look for side effects. Often a clinical trial is used to learn if a new treatment is more effective and/or has less harmful side effects than the standard treatment.
Pharmacovigilance and Risk Management
Risk management is a systematic approach to identifying, assessing and understanding, acting on, and communicating risk issues. All drugs have risks associated with their use, including adverse reactions, interactions between drugs, and the risk that the product may not work as effectively as expected a proactive approach to risk management of drug safety is dynamic throughout the whole life-cycle of a medicinal product.
-
Implementation of Risk Minimization Plans
-
Significant changes to Marketing Authorisation
Pharmacovigilance Significance & Scope
Pharmacovigilance, as defined by the World Health Organization, comprises the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects and other drug-related problems. The aim of pharmacovigilance developments to collect information about various broad aspects of medicinal product safety. These aspects are listed in the guidance file on good pharmacovigilance practices of the Food and Drug Administration (FDA). The revenue of this market is projected to growth at CAGR of 13% during the forecast period.
Drug Safety
Drug safety relates to the potential for adverse effects related to the administration of drugs. Efforts to establish the safely profile of drugs begin early in their development, with in vitro and in vivo toxicity testing, and continue through clinical trials leading up to drug approval and following approval in specific post-marketing studies or general pharmacovigilance efforts.
Drug absorption
Drug absorption is a pharmacokinetic parameter that refers to the way a drug is absorbed from a pharmaceutical formulation into the bloodstream. Drugs may be either weak acids or bases that exist in both ionized and non-ionized forms in the body. Drug in the non-ionized form is adequately soluble in membrane lipids and can cross cell membranes. The rate of absorption depends upon the ratio of the 2 forms at a particular site and is also a factor in circulation and elimination.
-
Pseudo-equilibrium
-
Mass Balance Approaches
-
Dynamic Models
-
Regulatory Aspects
Nano Pharmaceuticals
Nano pharmaceuticals offer the ability to detect diseases at much earlier stages and the diagnostic applications could build upon conventional procedures using nanoparticles .Nano pharmaceuticals are colloidal particles of 10 to 1,000 nano meters (1 micron) in size. They are widely used in drug delivery. Nano pharmaceutical reduces the cost of drug discovery, design & development and enhances the drug delivery process. As a result, the properties of nano materials are fundamentally different from those of their macroscopic/ bulk analogues due to an increased surface area and quantum effects. Nanomedicine for blood disorders
-
Nanodrugs for herbal medicines and cosmetics
-
Nano engineered drugs
-
Advantages of nanopharmaceuticals
Adverse drug reactions
Adverse drug reactions can be considered a form of toxicity .toxicity is most commonly applied to effects of over absorption. For information on toxicity of specific drugs see the table Symptoms and Treatment of Specific Toxins. Adverse drug reactions are usually classified as mild, moderate, lethal (see table Classification of Adverse Drug Reactions [ADRs]. Severe or lethal ADRs may be specifically mentioned in black box notices in the physician prescribing information provided by the manufacturer.
Drug Tolerance
Drug tolerance is revealing of drug use but is not necessarily associated with drug dependence or addiction. The process of tolerance development is reversible that can involve both physiological factors and psychological factors. Drug tolerance should not be disordered with drug tolerability, which refers to the grade to which over adverse effects of a drug can be tolerated by a patient.
Biopharmaceutics
Biopharmaceutics is a main branch in pharmaceutical sciences which relates between the physicochemical properties of a drug in dosage form and the toxicology, pharmacology or clinical response observed after its administration. It is not sufficient to know what the drug does to the body and it is also vital to know what the body does to the drug. The knowledge of the pharmacodynamic and pharmacokinetic properties of the drug.
Pharmacovigilance Practice
Good Pharmacovigilance Practices (GVP) begins with the acquisition of complete information from spontaneous adverse event reports. Good Pharmacovigilance Practice and Pharmacoepidemiology in Risk Management is mainly to increase the probability of beneficial effects of a drug in a population than the probability of adverse effects The pharmacovigilance and clinical trials services providing corporations ought to have the Pharmacovigilance certification.
-
Good reporting practices
-
Interpreting safety signals
Regulatory Efforts
Regulatory affairs experts play a significant role in communication between their company and different regulatory bodies such as the FDA, Singapore HSA, etc. We provide a wide range of regulatory affairs services, including assessing and repayment, to our client. There are several Regulatory Affairs departments depending upon the countries within ever growing pace of companies. Global Harmonization in standards has led to consistent approach in regulatory submissions and hence its review.
Pharmacoepidemiology
Pharmacoepidemiology combines clinical pharmacology with epidemiology. Pharmacology is the study of the effects of medicines in humans. It affects to using pharmacokinetics and pharmacodynamics of a patient to predict the drug effect on a patient. Pharmacoepidemiologists also conduct safety training of drug use in large populations. They are attentive in common, predictable adverse drug reactions as well as the uncommon and unpredictable ones.
-
Pharmacoeconomics
-
Biopharmaceuticals process validation
-
Role of pharmacovigilance and pharmacoepidemiology in risk management
Business Opportunity for Pharmacovigilance
Regulatory Affairs department is constantly evolving and growing and is the one which is least impacted during the Acquisition and Merger, and also during recession. The global Pharmacovigilance market and Business opportunity was valued at USD 2,408.0 million in 2013 and is expected to grow at a CAGR of 12.6% during the forecast period. Phase III clinical trials market was the second largest and was valued at over USD 750.0 million in 2013.
-
Career Growth in PV Consulting
-
Pharmacovigilance software Development Companies
-
Business Opportunities for PV Software Developing Companies
Pharmaceutical Toxicology
Pharmaceutical toxicology lies on the margin of the toxicology and drug discovery, and includes all the research about harmful effects and safety of medicines in relation to the therapeutic benefits. In adding and determination of the direct effect of the potential drug on the functioning of cells, tissues and organisms. Toxicologists can use computer models and in vivo tests to understand whether metabolism body is likely to affect the toxicity of the drug.
-
Safety assessment of pharmaceuticals
-
Safety Sciences of Drugs
-
Immunotoxicity
-
Biomedical Analysis
The global Pharmacovigilance (PV) and drug safety software market is expected to reach US$ 262.02 Mn in 2027 from US$ 151.07 Mn in 2018. The Pharmacovigilance (PV) and drug safety software market is estimated to grow with a CAGR of 6.4% from 2019-2027.
The market is driven by factors such as, rising incidences of adverse drug reactions (ADRs) and globalization of Pharmacovigilance. However, expensive technology may limit the growth of the market to a certain extent.
The Pharmacovigilance (PV) and drug safety software are used to recognize the cause of drug withdrawal and helps to inhibit unnecessary future events. These software are beneficial to those involved in drug making process like clinical research organization and pharmaceutical companies. These software help companies to manage huge data generated due to adverse drug reaction events. Help to maintain data for regulatory inspection and approval. These software lets global information to be shared rapidly and economically
The global Pharmacovigilance market size was estimated at USD 4.31 billion in 2018 and is anticipated to witness a CAGR of 13.3% over the forecast period. Increasing incidence of Adverse Drug Reactions (ADRs) is expected to accelerate the demand for Pharmacovigilance (PV) services.
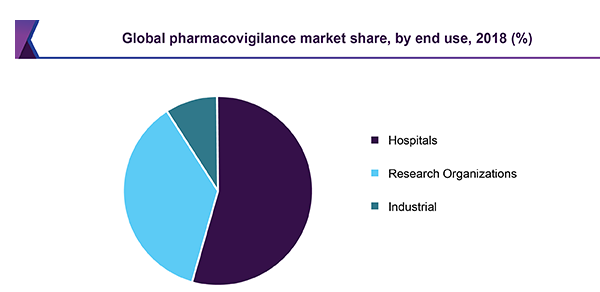
Growing prevalence of chronic diseases is another major contributor for the growth of the market. Treatment of these chronic diseases require uptake of combination of drugs resulting in ADR. PV services are used to curb this problem. According to the statistics published in the Journal of American Medical Association (JAMA), in 2014, ADR was one of the leading causes of mortality in U.S., resulting in more than 100,000 deaths every year.
Global Pharmacovigilance and Drug Safety Software Market: Overview
Increasing approvals for new molecules and biosimilars
New molecules and biosimilars undergo drug safety assessments before their launch in the market. This is to ensure that the drugs comply with safety standards as per regulations. This rise in approval of new molecular entities and biosimilars as well as registered activities of clinical trials globally has resulted in a surge in the adoption of Pharmacovigilance and drug safety software which will subsequently lead to the expansion of the global Pharmacovigilance and drug safety software market at a CAGR of close to 7% during the forecast period.
Outsourcing Pharmacovigilance and drug safety research to CROs
To reduce the operational costs and provide revenue-generating opportunities to the vendors, Pharmacovigilance, and drug safety operations are being outsourced to developing economies such as India, China, and the Philippines. This trend is expected to have a positive impact on the overall market growth.





